Volume 10 Issue 4
Olga Kozhar, Lydia Tymon, and Tobin L. Peever
Botrytis gray mold is the most economically significant fungal disease of red raspberry and other small fruit crops in Washington. The fungus Botrytis cinerea that causes gray mold is ubiquitous thriving in cool and humid environments, and gray mold outbreaks are often associated with rainy weather. Although cultural methods of disease control can help, the only effective tool to manage gray mold is through the application of fungicides. Since the fungus can infect crops at different stages of their growth and development, farmers are required to apply fungicides multiple times during the growing season. Frequent applications of fungicides creates strong selection pressure leading to the development of fungicide resistance. Once Botrytis develops resistance to specific active ingredients (a.i.) in these fungicides, they become ineffective in managing gray mold disease. Based on research performed by the WSU Small Fruit Pathology Team, widespread resistance exists to four of five fungicides registered for gray mold control in small fruit in WA. Such widespread resistance greatly limits disease control options for WA berry farmers who are in urgent need of new tools for disease management.
Pristine (a.i. = pyraclostrobin and boscalid) is one of the fungicides registered for management of gray mold on small fruit in WA. In 2014, resistance to the boscalid component of this fungicide was detected in 70% of WA small fruit fields surveyed. Boscalid belongs to Fungicide Resistance Action Committee (FRAC) Group 7 and is a site-specific chemical that disrupts respiration in fungal cells. Once the fungus absorbs the boscalid molecule, it binds to a specific enzyme in fungal cells, making the fungus incapable of producing energy and the fungus dies (Fig.1A). In order to survive in such an environment, the fungus makes genetic changes (mutations) in its genome that subsequently abolish binding to the target enzyme. This process leads to a mismatch between the enzyme and the boscalid molecule (Fig. 1B). As a result of these mutations, boscalid becomes harmless to the fungus and ineffective for gray mold control.
 Fig.1. Interaction between the boscalid molecule and the respiration enzyme in Botrytis is analogous to a “lock and key” principle. In boscalid-sensitive strains (A) the boscalid molecule (the “key”) matches the shape of the enzyme (the “lock”) and prevents the enzyme from working, while in boscalid-resistant strains (B) an interaction is no longer possible due to a change in shape of the binding enzyme and the enzyme still works in the fungal cells.
Fig.1. Interaction between the boscalid molecule and the respiration enzyme in Botrytis is analogous to a “lock and key” principle. In boscalid-sensitive strains (A) the boscalid molecule (the “key”) matches the shape of the enzyme (the “lock”) and prevents the enzyme from working, while in boscalid-resistant strains (B) an interaction is no longer possible due to a change in shape of the binding enzyme and the enzyme still works in the fungal cells.
Once Botrytis develops resistance to a fungicide from a certain FRAC group, other chemicals from the same FRAC group also become ineffective, a phenomenon known as cross resistance. However, recent studies have demonstrated that several newly developed FRAC Group 7 fungicides may provide control of boscalid-resistant strains even though these fungicides have similar chemistry and mode of action (Amiri et al. 2014, Olaya et al. 2016, Sierotzki and Scalliet 2013). Among these fungicides are “Kenja” (a.i. isofetamid), “Miravis Prime” (a.i. pydiflumetofen), ”Luna Tranquility” (a.i.s fluopyram and pyromethanil), “Fontelis”, (a.i. penthiopyrad), and “MerivonXemium”, (a.i. fluxapyroxad). Merivon Xemium is not currently registered for use on blueberry or red raspberry in WA (see WSU PICOL website for a current listing of registered products in Washington; current registrations are shown in Table 1). Whether a new FRAC group 7 fungicide will be effective against boscalid-resistant strains depends on the type of mutation in the Botrytis genome. To date, six different mutations have been detected in Botrytis worldwide that confer boscalid resistance. Fig.2 provides an overview of the relationships among FRAC Group 7 fungicides and boscalid -resistance mutations.
Table 1. FRAC Group 7 fungicides, active ingredients, field application rates, and cross- resistance among the FRAC group 7 fungicides based on mutations in the respiration enzyme. All fungicides are effective on non-mutant isolates . A check indicates when fungicide is expected to be effective against resistant Botrytis strains carrying corresponding mutation and an ‘X’ indicates when a specific mutation confers resistance to the corresponding fungicide and the fungicide is predicted to be ineffective in controlling gray mold in the field. a indicates a FRAC Group 7 active ingredient when there is more than one active ingredient in a product, b indicates when other active ingredient is FRAC group 11. Resistance to FRAC group 11 is known in Botrytis. c Fluxapyroxad is a FRAC Group 7 chemical that is not currently registered for use on blueberry or red raspberry in WA.
Columns 4-6 of Table 1 correspond to mutations H272R, H272Y, and H272V which have been detected in WA berry fields (Kozhar et al. 2020), with H272R and H272Y being the most prevalent. On average, 40% of boscalid-resistant strains in WA carry mutation H272R, and 60% carry mutation H272Y. All the new FRAC Group 7 fungicides are expected to be effective against Botrytis strains carrying the H272R mutation, while Kenja and Luna Tranquility are expected to be effective against strains carrying the H272Y mutation. Botrytis strains carrying the third mutation, H272V, confer cross resistance to all new FRAC Group 7 fungicides, but the frequency of this mutation remains extremely low in WA at this time. The frequency of this mutation needs to be carefully monitored in WA in the future.
In order to design effective gray mold fungicide application programs, WA berry farmers need to know which boscalid resistance mutations are present in their fields and choose the appropriate FRAC 7 fungicides that will be effective on their farm. Disease control programs will need to be planned carefully, alternating between active ingredients from different FRAC groups and decreasing the number of sprays per season to a minimum to reduce selection for resistance. WSU is currently developing two rapid and cost-effective methods for routine screening of Botrytis isolates from WA berry fields to help farmers get a better understanding of which resistance mutations are prevalent in their fields.
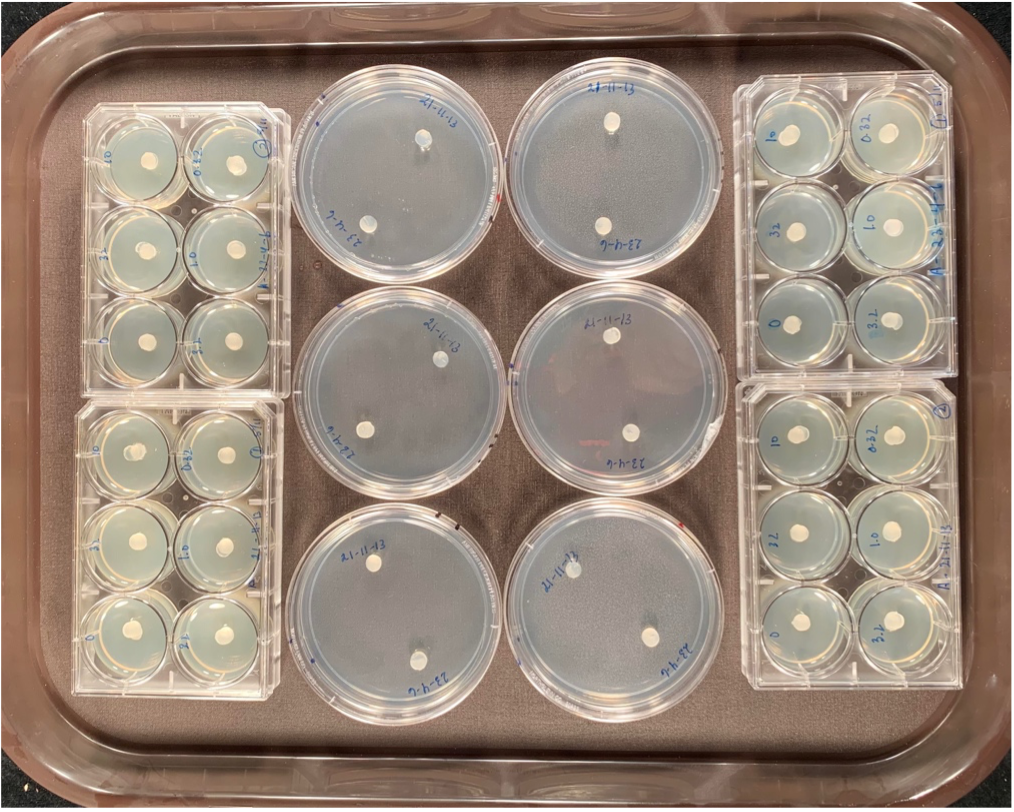
In the lab, Botrytis isolates traditionally have been evaluated for fungicide resistance by placing the fungus onto a plate amended with different concentrations of the fungicide (center of Fig. 2). One plate is amended with the fungicide, the other left as a control. Botrytis is then added to the plate (small circles on plates in Fig. 2). Growth of Botrytis at each concentration of fungicide is then measured after 2 days and compared to growth of the Botrytis isolate on a plate without any fungicide. One of the rapid or high-throughput methods is similar but allows for faster preparation time and uses less materials. The delivery of the fungicide to the plate occurs by saturating a sterile filter paper disk in the fungicide and placing it in the middle of one well of a miniaturized plate (left and right of Fig. 2). One disk is saturated with one fungicide concentration. An agar plug with Botrytis mycelium then is placed directly on top of the paper disk and growth is measured the same way as in the standard plate assay. Each 6-well plate is used to test one isolate on different fungicide concentrations which allows for accurate reading.
Fig. 2. Comparison of the traditional method (large plates in center) to the miniaturized method (outer rows left and right) to assess fungicide resistance in Botrytis. Left and right columns: Filter disks are saturated each with a concentration of the fungicide and Botrytis isolates are plated directly on top of the disk. Center column: Traditional plating method where fungicide is amended into the medium before Botrytis isolate plating.
In an article next month’s newsletter, we will explain DNA characteristics, how PCR or polymerase chain reaction works, as well as describe the second rapid method for detection of Botrytis mutations that confer fungicide resistance. This assay can detect differences in the Botrytis DNA leading to resistance without the need for plating the fungus as shown in Fig. 2.
References
Amiri, A, S.M. Heath and N. Peres. 2014. Resistance to fluopyram, fluxapyroxad, and penthiopyrad in Botrytis cinerea from strawberry. Plant Disease 98: 532-539.
Olaya, G., R. Linley, K. Edlebeck and T. Harp. 2016. ADEPIDYN fungicide: Cross resistance patterns in Alternaria solani. Abstract presented at the 2016 Annual Meeting of the American Phytopathological Society, July 30 to August 3, Tampa, FL.
Sierotzki, H. and G. Scalliet. 2013. A review of the current knowledge of resistance aspects for the next–generation succinate dehydrogenase inhibitor fungicides. Phytopathology 103: 880-887.
Kozhar, O., Larsen, M.M., Grünwald, N.J. and Peever, T.L. (2020) Fungal evolution in anthropogenic environments: Botrytis cinerea populations infecting small fruit hosts in the Pacific Northwest rapidly adapt to human-induced selection pressures. Applied and Environmental Microbiology, 9, e02908–e2919.